53+ use bond energies to calculate the heat of reaction:
Calculate the bond energy of the HI bond. Count how many of each bond is being broken.

Solved Question 12 Using The Following Data Reactions Ah Kjlmol Rxn 542 432 154 H2 G Fz G 2hf G H2 G 2h G F2 G 2f G Calculate The Energy Of An H F Bond A 44 Kj Mol B 271 Kj Mol
Web Using the values of bond dissociation energies calculate the heat of each reaction and determine whether it is an exothermic or endothermic process.

. H 2 g 2 H g Cl 2 g 2 Cl g Step 2 These atoms combine to form HCl molecules. Web HH II 2 HI The energy change for this reaction is 3 kJmol. Web This tutorial describes how to calculate the heat of a reaction involving covalent compounds using bond energies.
We break all the bonds to form atoms and then we reassemble the atoms to. Energy in 436 151 587 kJmol Energy out 2. Web Step 1 The reactant molecules H 2 and Cl 2 break down into their atoms.
Web To find the standard change in enthalpy for this chemical reaction we need to sum the bond enthalpies of the bonds that are broken. Web Use bond energies to calculate the heat of reaction. Web Steps for Using Bond Energies Lewis Structures to Calculate the Heat of Reaction Step 1.
Look up bond energies. Web with these average bond energies involved. Web Use bond energies provided below to calculate the heat of reaction.
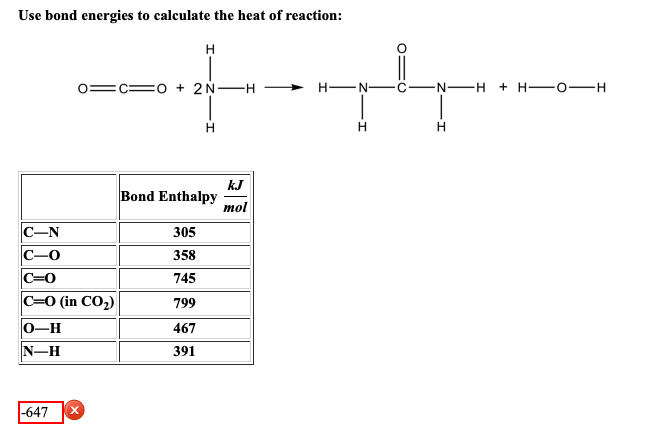
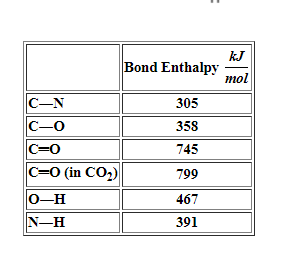
We break all the bonds to form atoms and then we reassemble the. The product of this reaction is eq2 H_2O eq. H oCO 2N-H H-N -N-H H-0-H H H H kJ Bond Enthalpy mol C-N 305 C-0 358 C0 745 CO in CO2 799 0-H 467 N-H.
Web We use Hesss Law when we use bond energies to calculate heats of reaction. Calculate enthalpy change or heat of reaction. Web We use Hesss Law when we use bond energies to calculate heats of reaction.
And from that we subtract the sum of the. For example let calculate the C-H bond. Web Define bond energy.
For the last reaction. - Well provide some tips to help you choose the best Use bond energies provided below to. Web Bond energies may also be calculated from appropriate data obtained from calorimetric and other measurements and use of Hesss law.
For a given reaction can be calculated using the bond energy values from Table. H H 105 kcalmol O O 119 kcalmol O H 110 kcalmol In this reaction 2 HH bonds and 1 OO bonds are. Determine the bond enthalpy of the products eqH_ products eq by using a bond enthalpy table.

Relativistic Heavy Neighbor Atom Effects On Nmr Shifts Concepts And Trends Across The Periodic Table Chemical Reviews
Solved Use Bond Energies To Calculate The Heat Of Reaction Chegg Com
Answered Use Bond Energies To Calculate The Heat Bartleby
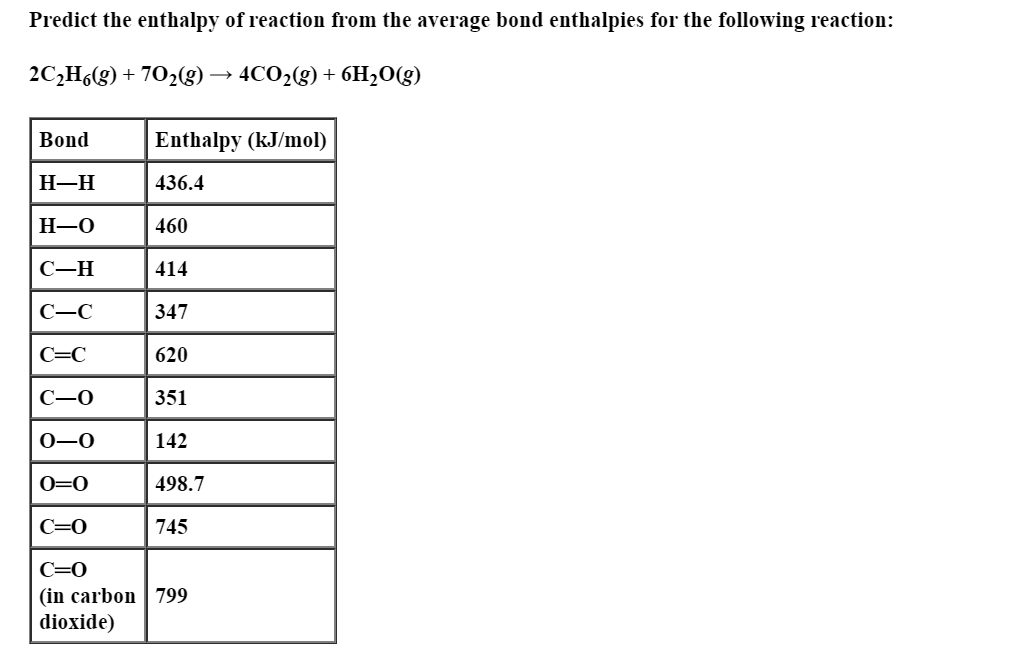
Answered Predict The Enthalpy Of Reaction From Bartleby
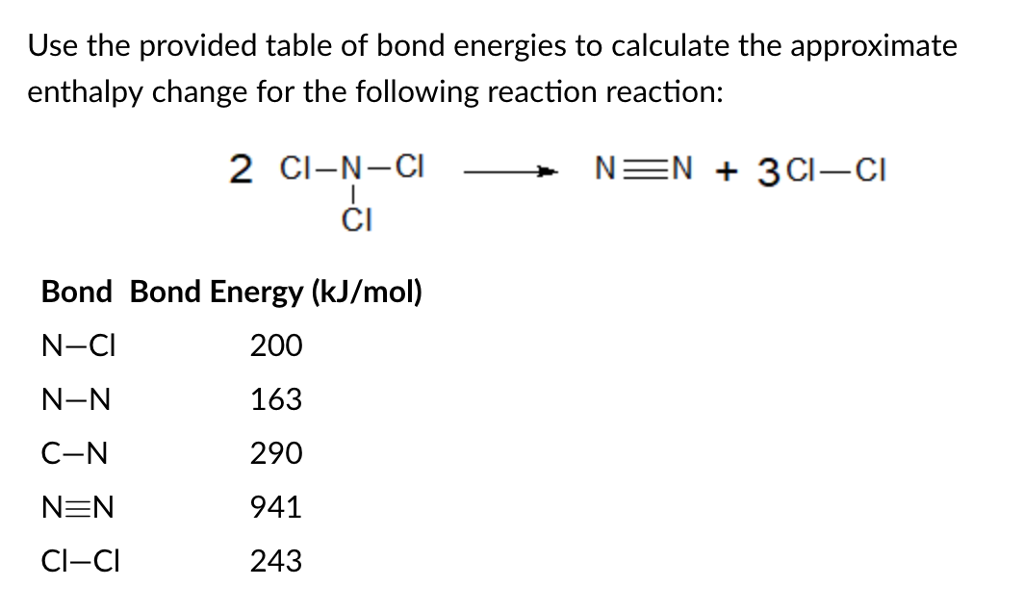
Solved Use The Provided Table Of Bond Energies To Calculate Chegg Com

Feofilov Artem 74784 Textethesecr

A Dependence Of Fluorescence Intensity Of Bi 5 Gacl 4 3 A Download Scientific Diagram
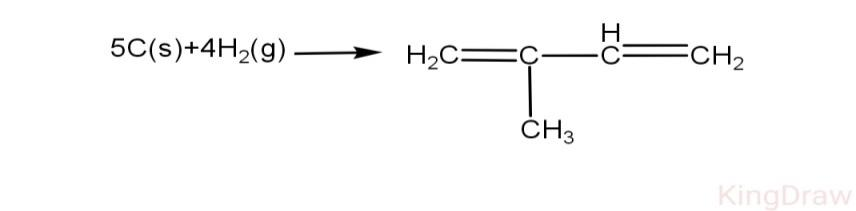
Using Bond Energy Data Calculate Heat Of Formation Of Isoprene Given C H H H C C C C And C S To C G Respectively As 98 8 Kcal 104 Kcal 83kcal 147kcal 171kcal N N N N N A 21

How To Use Bond Energies Lewis Structures To Calculate The Heat Of Reaction Chemistry Study Com

Wo2021021417a1 Hydrocarbon Polymer Modifiers Having High Aromaticity And Uses Thereof Google Patents

Aleks Calculating The Heat Of Reaction From Bond Energies Youtube
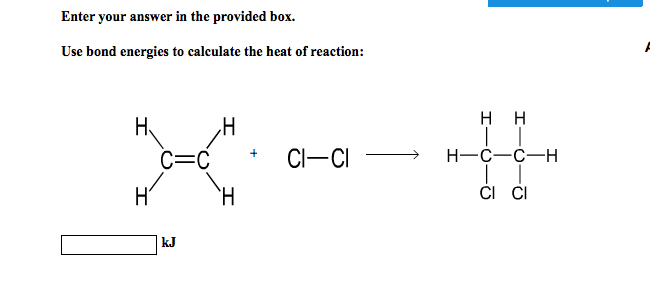
Solved Enter Your Answer In The Provided Box Use Bond Chegg Com

Enthalpies Of Reactions Using Average Bond Enthalpies Chemistry Tutorial Youtube

Chemical Energy 07 12 Ppt Download
Chemical Energy Examples Pdf Examples

Objectives Understand That Chemical Reactions Involve The Making And Breaking Of Bonds And The Concept Of Bond Enthalpy Be Able To Determine Bond Ppt Download

Bond Energies Worksheet Bond Energies 1 2 3 4 5 Read Pages 586 587 In Your Textbook Which Process Releases Energy Breaking A Bond Or Forming A Course Hero